For the process of saponification to occur, all the ingredients must be in liquid form (this includes all of the oils, fats and the alkali). For this reason, we use water in the soap formulation to dilute the alkali, and the oils or fats (those that are solid at room temperature) should first be molten before the process can begin.
The alkalis used for soap making are Sodium Hydroxide (chemical formula: NaOH), and Potassium Hydroxide (chemical formula: KOH). Both NaOH and KOH are solids (pearl / flake and sometimes powder) when supplied in their concentrated form. When chemists talk of “alkali”, they generally refer to Sodium or Potassium Hydroxide. When we talk of “caustic soda”, we usually refer to the same thing – whereas a “caustic solution” or “lye solution” is the strongly alkaline liquid made by dissolving either pure NaOH or KOH in water. Soap has distinct properties dependant upon the nature of the alkali used in their production, Sodium Hydroxide (NaOH) gives “hard soap” . Whereas, when Potassium Hydroxide (KOH) is used, a “soft soap” is formed.
The process of making soap is called saponification. It is the chemical reaction between a fat (acid) and the NaOH (base) to produce a salt (soap) as per illustration below
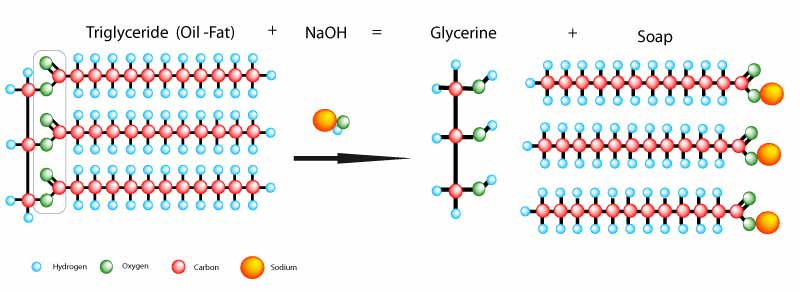
How does this reaction take place.
How exactly does reaction take place? In order to understand it, you must consider the chemical makeup of the acid and base being used in the reaction.
The base must always be composed of at least one hydroxide ion (OH–). For this reason most people use lye (NaOH) as their base.
There are many different types of fats and Oils that will react with Sodium or Potassium Hydroxide and saponify. Your Oil could be Olive Oil, Coconut Oil or PalmKernel Oil just to name a few.
Each Fat / Oil (triglyceride) has a unique combination of different length fatty acids. The amount of NaOH (or KOH) needed to react with the oil will vary depending on the chemical makeup of the various acids. (Tryglycerides; are compounds made of three fatty acids, attached to a single molecule of glycerol) which combines with the base (lye /NaOH) differently. We have also created a general use saponification table that can be download and used.
As you combine the reactants(fat and lye), and stir they start to react. The triglycerides release the single glycerol molecule (which turns into glycerine) and allows the three fatty acids (simultaneously released) to combine with the hydroxide ions within the base, thus forming the soap.
Click here to learn more about fatty acids and soap making.



